Abstract
Introduction: Myeloproliferative neoplasms (MPN) are frequently associated with symptoms of fatigue, poor sleep, mood disorders, lightheadedness and shortness of breath. The cause of these symptoms is not well understood and is often attributed to blood count abnormalities or elevated inflammatory cytokines common in these disorders. There is a high degree of overlap between clinical manifestations of MPN and symptoms reported by pts with pulmonary hypertension (PH). Small studies have shown that pts with MPNs are at risk for PH but the extent to which PH contributes to clinical symptoms and prognosis in MPN pts is unknown.
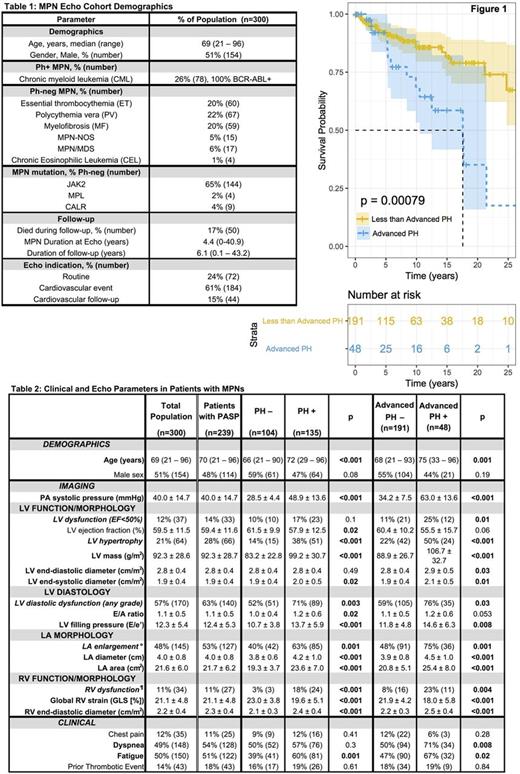
Methods: 300 sequential MPN pts undergoing transthoracic echocardiography (echo) between January, 2011 and April, 2017 were studied (Table 1). A second MPN cohort of 287 patients (NoEcho) was matched for age and diagnosis to the MPN Echo+ (Echo) cohort. Baseline demographics including age, gender, diagnosis, date of diagnosis, mutation profile, transformation, BMT, bleeding or thrombosis history, symptoms, cardiovascular risk factors, echo indication and survival were collected for all pts. To ensure accurate echo assessment of PH sequelae, RV assessment via both tricuspid annular plane systolic excursion (TAPSE) and peak tricuspid annular systolic velocity (S') were required for study inclusion. RV dysfunction (RVDYS) was defined by impairment of both TAPSE and S'. RV global longitudinal strain (GLS) was quantified as a secondary means of assessing RV function as a continuous variable. Pulmonary artery systolic pressure (PASP) was calculated based on tricuspid regurgitant velocity and inferior vena cava caliber; PH (PASP³35 mmHg) and advanced PH (PASP³50 mmHg) were defined via established criteria. LV systolic and diastolic function, geometry, and mass were quantified based on linear dimensions in parasternal long axis, consistent with quantitative methods previously validated. Echoes were interpreted in a high-volume laboratory, for which expertise and reproducibility have been documented. All cause mortality was assessed following echo.
Results: PH was identified in the majority (56%; 135/239) of MPN pts who underwent echo (Table 2). PH was common among all MPN subtypes (46% CML, 58% ET, 57% PV, 63% MF, 100% CEL, 42% MPN/MDS and 79% MPN-NOS). Advanced PH was identified in 20% of all MPN pts and within each subtype (15% CML, 13% ET, 16% PV, 29% MF, 33% CEL, 25% MPN/MDS and 43% MPN-NOS). RVDYS was 8-fold more common (24% vs 3%; p=<0.001) among MPN pts with PH and the magnitude of RVDYS increased stepwise in relation to PASP (p<0.001), suggesting that PH was causally related to RV failure. Although impaired LV systolic function (LVEF<50%) was no more common in MPN pts with PH (17% vs 10%; p=0.10), LV hypertrophy (38% vs 14%, p=<0.001), LV mass (99 ± 31 vs 83 ± 23 g/m2, p<0.001), LV filling pressure (E/e': 14±6 vs 11±4; p<0.001), and frequency of LV diastolic dysfunction (any grade: 71 vs 52%; p=0.003) were all increased in pts with PH. Left atrium (LA) area was also increased in MPN pts with PH (24 vs 19 cm2; p=0.001), further suggesting a post-capillary cause for PH in this MPN population. Myocardial biopsy of an MPN patient with advanced PH demonstrated interstitial fibrosis in non-infarcted myocardium suggesting that fibrosis could play a role in LV diastolic dysfunction and PH in pts with MPNs.
During median follow-up of 6.2 yrs (0.1 to 43 yrs), likelihood of death was higher for pts with advanced PH (odds ratio 3.1, p=0.004), or with RVDYS whether quantified by GLS (odds ratio 2.4, p=0.04) or TAPSE/S' (odds ratio 2.3, p=0.048). Cox Proportional Hazards model (parameterized for age, gender, MPN diagnosis, transformation and BMT) indicated that the Echo cohort was similar to the NoEcho control group. Pts with PH were more likely to experience fatigue than pts with a normal estimated PASP. Whereas dyspnea was no more common in pts with PH as a whole, pts with advanced PH were more likely to experience both fatigue and dyspnea compared to those with less severe PH or normal PASP. Cox proportional hazards modeling identified Advanced PH as an independent predictor of death in models that included Age, Gender, MPN Diagnosis, Transformation and BMT.
Conclusions : PH with post-capillary hemodynamics is extremely common in pts with MPN and is associated with frequently reported symptoms such as fatigue. When advanced or when associated with RVDYS, PH independently confers increased mortality risk for MPN pts.
Ritchie: Bristol-Myers Squibb: Other: Research funding to my institution; Astellas Pharma: Other: Research funding to my institution; Celgene: Consultancy, Other: Travel, Speakers Bureau; Pfizer: Consultancy, Other: Research funding to my institution; NS Pharma: Other: Research funding to my institution; Incyte: Consultancy, Speakers Bureau; Novartis: Consultancy, Other: Research funding to my institution, and travel, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.